Data & Access
Overview
Anyone can request access to the Cancer Alliance Queensland data holdings - researchers, clinicians (public or private), hospital administrators, policy makers and the public.
The Cancer Alliance Queensland has a dedicated Cancer Control Information team who will assign a team member to your data request to act as your main point of contact. This team is responsible for processing cancer data access requests and providing data in accordance with legislative provisions.
The information below outlines steps on how to request data.
Cancer Data Explorer
The Cancer Data Explorer is a dashboarding tool allowing users to access information on the number of new cases of cancer (incidence), deaths due to cancer (mortality), cancer survival, projections and prevalence among Queenslanders with cancer. The data is sourced from the Queensland Cancer Register, a database containing information on Queenslanders diagnosed or dying from cancer since 1982. The tool is freely available to the general public, researchers, clinicians, policy makers and government bodies alike.
Research ready data
Research ready data supports state-wide retrospective analyses and is an initiative of the Queensland Cancer Control Safety and Quality Partnership (a gazetted quality assurance committee).
These data sets contain unit record data elements which have been curated, clinically reviewed, and internally approved for release by Cancer Alliance Queensland in a format that can be used for analysis.
Each research ready data set has a technical appendix which defines the data elements. To find out more contact us.
Request data for research
I am wanting to conduct a research project with the aim to publish results.
Consultation with the Cancer Control Information team prior to applying for research ethics approval, grant applications or publication is strongly recommended to ensure that the relevant data is available and that there are adequate resources to be able to provide the data required.
Follow the STEP BY STEP GUIDE on how to request data for the purpose of research and the approvals required under legislation and privacy laws.
Define the type of data required for your research
|
Step 1 |
Non-identifiable aggregated dataThis information is used to produce public reports and is available for tailored analysis using our online statistics tool OASys or OASys data tables.
Read more
Non-identifiable aggregated data is grouped by non-identifiable data elements for example age group, sex, cancer site, diagnosis year and is available in the public domain. For example 5 year age groups for Indigenous women diagnosed with cervical cancer in Queensland. For customised non-identifiable aggregated data requests Read more
Confidential data: can be potentially identifiable or identifiable
Potentially identifiable unit record information This data does not contain name, address, date of birth or individual facility but may include data elements that either singly or used in combination identify an individual eg.country of birth, Indigenous status, postcode, age or if a researcher uses a way to re-identify an individual uniquely eg. URN, QOR Patient ID. Identifiable unit record information This data does not contain name, address, date of birth or individual facility but may include data elements that either singly or used in combination identify an individual eg. country of birth, Indigenous status, postcode, age or if a researcher uses a way to re-identify an individual uniquely eg. URN, QOR Patient ID. Files containing individually identified case record information that includes any personal identifiers which enable a person to establish the identity of a person or organisation. Examples of identifiable data elements are name, address, date of birth
|
|
plan your Research & write A PROTOCOL |
Step 2 |
|
Complete your own study protocol or download the Cancer Alliance Queensland study protocol template.
|
|
submit a data request to cancer alliance Queensland |
Step 3 |
|
Download and complete the Cancer Alliance Queensland Data Request Form for Research. Complete the online Cancer Alliance Queensland data request registration. (This is where you upload the completed Data request form for research (above) and submit the registration with your research project supporting documentation (study protocol, ethics application/approval). A member of the Cancer Control Information team will contact you to confirm data availability and provide advice on the approval pathway based on the type of data request specific to your data specifications and mode of delivery requested.
|
|
Submit an ethics application and site specific assessment for approval |
Step 4 |
|
All research studies must have a protocol, obtain Human Research Ethics Committee (HREC) approval and have Site Specific Assessment (SSA) approval. To complete a Human Research Ethics Application go to the Online Forms website. Submit the HREA to your chosen HREC Committee and follow their instructions for approval. (List of HREC Committees) You will also be required to complete and submit a Site Specific Assessment with the HREA. A standard template is available on the Online Forms website. This process deals primarily with research budgets, funding sources, recruitment, human resources, contracts/agreements and local site policies and will require approval prior to undertaking research. For further information for researchers go to the Office of Research and Innovation (ORI) website. Skip this step if your research has obtained HREC and SSA approval.
|
|
submit a public Health APPLICATION for data custodian approval |
Step 5 |
|
All research studies must seek data custodian approval. This ensures that the relevant data is available for your research and there are adequate resources to the Data Custodian available to provide the data required. Download and complete the Public Health Application (PHA) Information & Application form. When completing this form you must document all the data elements, inclusion or exclusion criteria and explain the of method of delivery. These details will assist the Cancer Control Information team with data extraction and methods of delivery. If your research requires Multi Disciplinary Team (MDT) information that is recorded in QOOL the Data Custodian requires a MDT Chair Declaration form to be submitted with the PHA application. If your research will be contacting patients the patient information sheet and consent form must also be provided with the PHA. Send the completed PHA application, the HREC approval and/or the completed MDT Chair Declaration form to the Cancer Control Information team for the Data Custodian to approve. Once your PHA is signed by the Data Custodian, a copy of the signed PHA will be emailed to you.
|
|
Submit the data custodian approved Public Health application to the Department of Health for approval |
Step 6 |
|
You must apply to the Director-General of the Department of Health or his/her delegate for access to health information held by the Department of Health. You are required to send the Data Custodian approved PHA application with HREC approval by email to the Office of Research and Innovation (ORI). HIIRO will submit your application to the Director-General/Delegate for approval and if approved, an approval letter for the release of data for your project will be sent to you.
|
|
notify Cancer Alliance Queensland of approval |
Step 7 |
|
Email the approval letter to the Cancer Control Information team to organise the release and transfer of approved data for your research.
|
Request Data for Clinical Audit
|
I work for the Queensland Department of Health and need some data for the purpose of planning and evaluation of health services. If I do not work for the Queensland Department of Health or I am not a 'designated person' under section 139A of Hospital and Health Boards Act 2011, legislation prevents the release of data under these circumstances. Go to request data for research and follow steps for requesting data and approvals. What type of data can be requested?
What Cancer Alliance Queensland data holdings are available:
Examples of clinical audits:
Follow the STEP BY STEP GUIDE on how to request data for the purpose of clinical audit if I work for the Queensland Department of Health or I am a 'designated person'.
|
Define the type of data required and intended use
|
Step 1 |
|
Please note that this is limited to Queensland Department of Health employees or other organisations authorised by legislation to access potentially identifiable or identifiable data for non-research purposes. Data can be released to a ‘designated person’ for evaluating, managing, monitoring or planning health services under section 150 of the Hospital and Health Boards Act 2011.
Confidential data: can be potentially identifiable or identifiablePotentially identifiable unit record information.This information is regarded as confidential and is protected by legislation.
Read more
This data does not contain name, address, date of birth or individual facility but may include data elements that either singly or used in combination identify an individual eg. country of birth, Indigenous status, postcode, age or if a researcher uses a way to re-identify an individual uniquely eg. URN, QOR Patient ID. Identifiable unit record information. This information is regarded as confidential and is protected by legislation. Files containing individually identified case record information that includes any personal identifiers which enable a person to establish the identity of a person or organisation. Eg name, address, date of birth |
|
plan your Clinical Audit & write A PROTOCOL |
Step 2 |
|
If your intention is to ‘do research’ in any form then appropriate ethical review and approval is required follow the request data for research step by step guide. Contact the Cancer Control Information team to ensure that the data you require is available. Complete your own study protocol or download the Cancer Alliance Queensland study protocol template. |
|
submit your data request to cancer alliance Queensland |
Step 3 |
|
Download and complete the Cancer Alliance Queensland Data request form for Clinical Audit or HREC Exemption. (If your audit is for a project that does not meet the definition of research, such as routine service evaluation but contains information that may be relevant for publication, a letter from an ethical committee is often requested by peer-reviewed journals). You must request a letter from the HREC which states that the project is exempt from formal ethical review due to the nature of the project and provide this letter with your application to the Cancer Alliance Queensland. Complete the online Cancer Alliance Queensland data request registration. (This is where you upload the completed Cancer Alliance Queensland Clinical Audit or HREC Exemption form (above) and submit registration with supporting documentation (study protocol, HREC exemption approval letter). A member of the Cancer Control Information team will contact you to confirm data availability & provide advice on the approval pathway based on the type of data request specific to your data specifications and mode of delivery requested.
|
Request Data for My MDT
|
I am a member of a Multidisciplinary Team Meeting and would like some data for the purpose of evaluating, managing, monitoring or planning my MDT. Members of MDT's can access MDT data via QOOL-Dash. If QOOL-Dash does not contain the information you require, follow the steps below. What type of data cancer be requested?
What Cancer Alliance Queensland data holdings are avaiable:
Examples of My MDT data requests:
Follow the STEP BY STEP GUIDE on how to request data for my MDT and the approvals required under legislation and privacy laws. If you are a member of the MDT and wish to review data on those patients for whom you are currently in a treating relationship with and for the specific purpose of evaluating, monitoring or planning your MDT then log on to QOOL-Dash OR simply send an email to qoolsupport@health.qld.gov.au and outline the data you require. When you send in your request, make sure you explain:
A member of the QOOL team will contact you to discuss your request. Following data custodian approval the data will be provided to you by Queensland Department of Health email in a password protected file. |
Request Data for My Personal Information
|
I am a cancer patient wanting to access or amend my personal information. Individuals can apply for access to documents held by the Queensland Government under the Right to Information Act 2009 (QLD)(RTI Act) and the Information Privacy Act 2009 (QLD)(IP Act). These Acts outline privacy principles and allows individuals to apply for access and amendment of personal information. To apply for access to, or to amend your information held in the Cancer Alliance Queensland data holdings go to Make a Right to Information request. |
Quality Assurance
|
The Partnership operates as an approved Quality Assurance Committee (QAC). This allows the Partnership to facilitate the participation of clinicians and administrators responsible for the management and delivery of cancer services, in the peer review of the safety and quality of cancer services and service improvement. The QAC gazettal allows the Partnership to access identifiable information and use it to better understand the safety and quality of the care services delivered across Queensland. This data is collected in accordance with the quality assurance guidelines under Part 6, Division 1 of the Hospital and Health Boards Act 2011. The data can only be used to fulfil the functions of The Partnership according to the Terms of Reference, therefore there is no data request process available. Examples of reporting:
For a full list of publicly available reports, view our Reports and Publications. If you would like to know more about The Partnership activities contact us. What type of ata cancer be requested?
Available Cancer Alliance Queensland data holdings:
Examples of quality assurance activities:
|
Statistics & Reports
|
Cancer Alliance Queensland has developed diverse and robust state-wide clinical oncology datasets that can be made available for quality assurance activities, research, health service planning, and to policy makers and the public. Our systems allows users to translate and analyse de-identified data from the Queensland Oncology Repository that aims to provide information to improve overall cancer care. Cancer Alliance Queensland analyses and reports on cancer and cancer care in Queensland with a focus on evidence based reporting. Our findings are published as reports, peer-reviewed journal articles and are presented at conferences. In addition to routine reporting, we provide reports that help to answer specific questions posed by clinical teams, health system stakeholders and policy makers. These reports inform the development, implementation and evaluation of cancer services in Queensland. Our reports are highly valued at a state and national level. View our Reports & Publications |
||
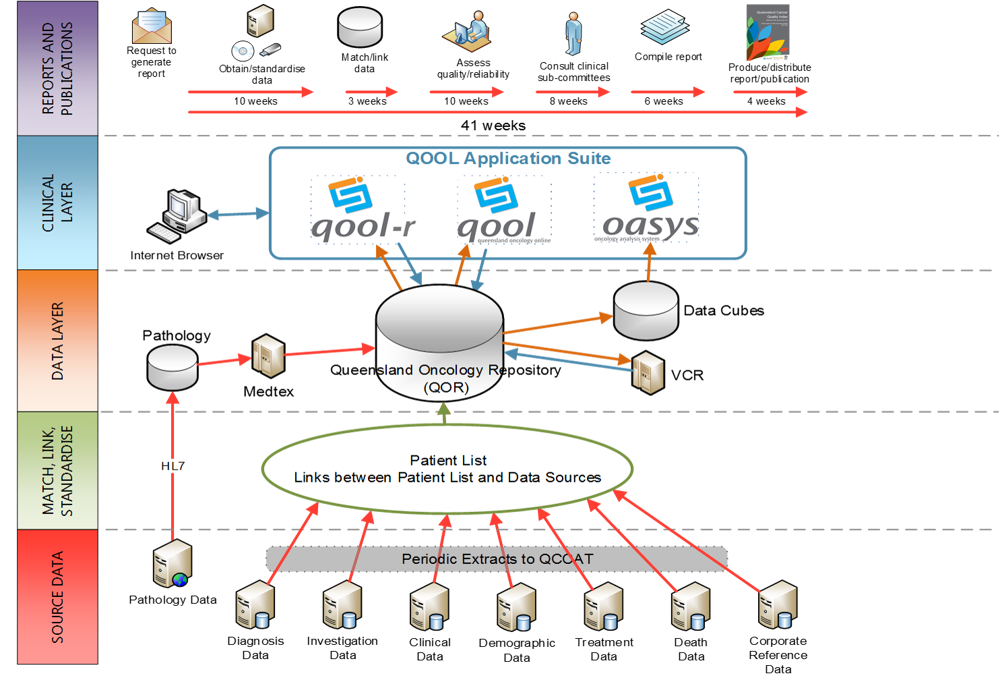
Big Data
Cancer Alliance Queensland is continually building a solid information management infrastructure, capable of supporting a fully integrated centralised oncology repository, known as the Queensland Oncology Repository (QOR), which is the largest of its kind in Australia. Together with advances in information technology and improvements to the Queensland Cancer Register, QOR provides comprehensive high quality data for the analysis, monitoring and evaluation of cancer services that supports clinicians, policy makers, health system planners and researchers, to improve the performance of cancer services.
This centralised repository holds information collected from over 60 data systems and cancer care providers across Queensland. The Cancer Alliance Queensland works collaboratively with the data custodians and cancer care providers to keep the data up to date and relevant.
It is important to highlight that for data to be useful in QOR, it must be routinely and accurately collected by stakeholders in order to provide a detailed population wide view of cancer in Queensland for the cancer community.
QOR contains approximately 46 million clinical records and holds data from over 60 data source systems across Queensland. Key to our program of work is our ability to link population-based cancer information from multiple sources. Our matching and linking processes provide over 600,000 matched and linked individual cancer patient records.
Once the data has been matched and linked in QOR the data is integrated into a data warehouse which is used for reporting and data analysis. The QOR data warehouse is secure, reliable, easy to retrieve and easy to manage allowing data consolidation, analysis and reporting at different aggregate levels. Data is transformed to match a uniform data model, cleansed of duplicates and inaccuracies, and is extracted with business intelligence and reporting tools. The QOR data warehouse contains the entire scope of data and can be used for both aggregate and very specific analysis and reporting. Business rules are created to transform the data and the development of these rules is undertaken in conjunction with our Sub-committee working groups and through clinical peer review. The data warehouse allows us to develop diverse and robust state-wide clinical oncology datasets for cancer control stakeholders. This dataset is known as Queensland Oncology Repository-Discovery data.
Cancer Alliance Queensland Data Infrastructure
Tech Talk & Security
Cancer Alliance Queensland is leading the way by aligning with the state-wide eHealth strategy of a patient centric focus of health care delivery across a networked model of care. This state-wide clinical registry provides linked patient information allowing clinicians to participate more effectively in audit, peer review and feedback activities as part of routine clinical practice.
Our purpose-built collection, matching and linking engine ingests and collates over 46 million records from hundreds of data sources across both public and private health services.
We use a range of leading industry platforms to power our collection of services:
- our data platform is backed by the powerful Microsoft SQL Server 2016
- we do enterprise level data warehousing with SQL Server Analysis Services 2016 which allows our talented stats team to do rich reporting and data visualization
- statistical analysis using Stata v13 and Microsoft Visual Studio 2017 and Microsoft Excel
- mapping and geographic analysis using ABS geography data and Google Maps for Business
- we craft and finely tune our suite of applications and services using the Microsoft .Net Framework and modern Javascript front-end frameworks
Security Measures
We take security seriously and we align with the e-Health security policies and follow industry best practices to protect our systems and data. Users can have different roles within our applications and are granted access only to data appropriate to their role.
Our data holdings are collected in accordance with the Quality assurance committees under Part 6, Division 1 of the Hospitals and Health Boards Act 2011.
Useful links & resources
